
Whole Lotta Rules Going On
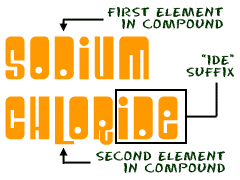
The process of naming compounds is just a set of rules. We're going to show you some of the basics. There are some advanced ways of naming things that we're going to skip right now.When you have two different elements, there are usually only two words in the compound name. The first word is the name of the first element. The second word tells you the second element and how many atoms there are in the compound. The second word usually ends in IDE. That's the suffix. When you are working with non-metals like oxygen (O) and chlorine (Cl), the prefix (section at the beginning of the word) of the second element changes based on how many atoms there are in the compound. It's like this...
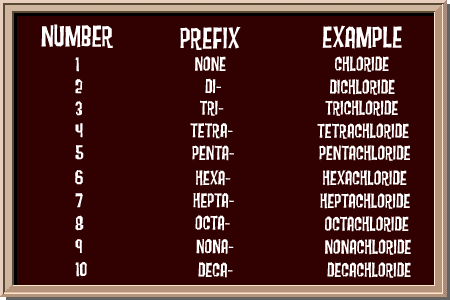
Do you notice anything about the chalkboard? You can see that the prefixes are very similar to the prefixes of geometric shapes. You know what a triangle is. Right? Well the prefix tri- means three. So when you have three chlorine atoms, you would name it trichloride.
 Look at the other names too. You may know about a pentagon, a hexagon, or an octagon. The naming system in chemistry works the same way!
Look at the other names too. You may know about a pentagon, a hexagon, or an octagon. The naming system in chemistry works the same way!
Let's put these ideas together! Remember, we're only talking about simple compounds with no metal elements. Most simple compounds only have two words in their names. Let's start with carbon monoxide (CO). That name tells you that you have one carbon (C) atom and one oxygen (O) atom (you can also use the prefix MONO to say one atom). Remember that the second word ends in -ide. So...
(1) Carbon + (1) Oxygen = Carbon monoxide (CO)
Now we'll build on that example. What if you have one carbon (C) and two oxygen (O) atoms?
(1) Carbon + (2) Oxygen = Carbon dioxide (CO2)
One last example and we'll call it quits. Now you have one carbon (C) and four chlorine (Cl) atoms.
(1) Carbon + (4) Chlorine = Carbon tetrachloride (CCl4)
You should be getting the idea now. The compound name can tell you how many atoms are inside. Take a look at some of the examples and see if you understand what is happening in the name.
Related Video...
Curiosity Collects Samples (NASA/JPL Video)
Encyclopædia Britannica: Chemical Nomenclature
Wikipedia: Chemical Nomenclature
Encyclopedia.com: Compounds



