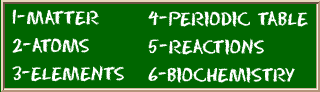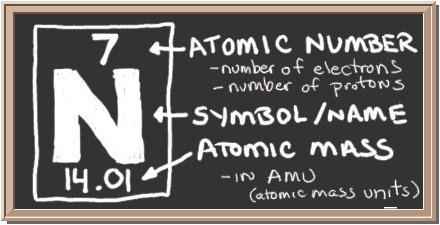
Check out the blackboard. That box on the left has all of the information you need to know about one element. It tells you the mass of one atom, how many pieces are inside, and where it should be placed on the periodic table.
In the next section we're going to cover electron orbitals or electron shells. This may be a new topic to some of you.
Electrons In The Shells
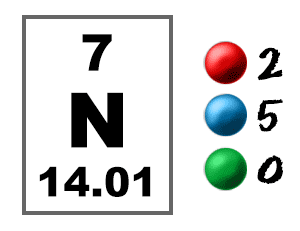
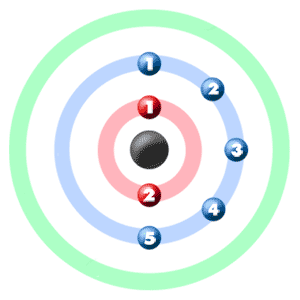
Take a look at the picture below. Each of those colored balls is an electron. In an atom, the electrons spin around the center, also called the nucleus. The electrons like to be in separate shells/orbitals. Shell number one can only hold 2 electrons, shell two can hold 8, and for the first eighteen elements shell three can hold a maximum of eight electrons. As you learn about elements with more than eighteen electrons you will find that shell three can hold more than eight. Once one shell is full, the next electron that is added has to move to the next shell.So... for the element of NITROGEN, you already know that the atomic number tells you the number of electrons. That means there are 7 electrons in a nitrogen atom. Looking at the picture, you can see there are two electrons in shell one and five in shell two.
Examples of Compounds with Nitrogen
AmmoniaThis is an ammonia molecule with the formula, NH3. Scientists use the name "ammonia", the same way they call H2O "water". In this compound, three hydrogen (H) atoms share their electrons with the nitrogen (N) atom. This way the nitrogen has a filled outer shell and the hydrogens have two electrons to fill their shells. |
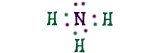 |
|
|
|
||
Nitrogen TrichlorideNitrogen can combine with three chlorine (Cl) atoms, forming nitrogen trichloride, or NCl3. Nitrogen shares its electrons with the chlorine atoms, so all of the atoms have their shells filled.Take a look at the dots around the atoms. All of them now have eight electrons, and a filled outer shell! |
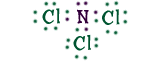 
|
|
|
|
||
Cyanogen ChlorideHere's something new! We have three different elements here, carbon, nitrogen, and chlorine. That's not special, but the way they combine is! Look at the carbon and the nitrogen, they are sharing six electrons!When two atom share two electrons, that's a single bond. If they share four it's a double bond. Well these two are sharing six, that's a triple bond. It's extremely strong and powerful. It would take a lot of work to separate the C and the N! One more thing! Because the bond between carbon and nitrogen is so strong, scientists call them "cyanogen" instead of carbon-nitrogen. Scientists know that cyanogen is always CN. |
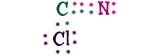 |
|