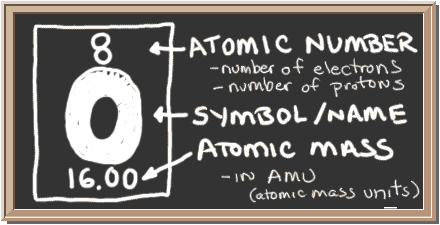
Check out the blackboard. That box on the left has all of the information you need to know about one element. It tells you the mass of one atom, how many pieces are inside, and where it should be placed on the periodic table.
In the next section we're going to cover electron orbitals or electron shells. This may be a new topic to some of you.
Electrons In The Shells
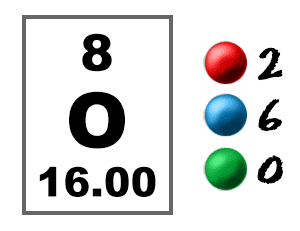
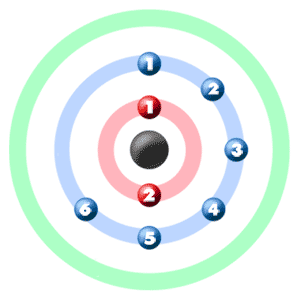
Take a look at the picture below. Each of those colored balls is an electron. In an atom, the electrons spin around the center, also called the nucleus. The electrons like to be in separate shells/orbitals. Shell number one can only hold 2 electrons, shell two can hold 8, and for the first eighteen elements shell three can hold a maximum of eight electrons. As you learn about elements with more than eighteen electrons you will find that shell three can hold more than eight. Once one shell is full, the next electron that is added has to move to the next shell.So... for the element of OXYGEN, you already know that the atomic number tells you the number of electrons. That means there are 8 electrons in an oxygen atom. Looking at the picture, you can see there are two electrons in shell one and six in shell two.
Examples of Compounds with Oxygen
WaterThis is a water molecule. Even though you find complex names on other molecules, everyone calls H2O "water". Water is made up of two hydrogen (H) atoms and one oxygen (O) atom. The formula for water is H2O. The hydrogen atoms have filled orbitals with two electrons and the oxygen atom is filled with eight electrons. |
|
|
|
|
||
Lithium OxideTwo lithium (Li) atoms can bond with one oxygen (O) atom, making the formula Li2O. Oxygen likes to have two additional electrons to make it happy. Each lithium atom provides one. You can see that the Oxygen atom has eight electrons (6 of its own, and one from each lithium), and the two lithium atoms have two electrons each. |
|
|
|
|
||
Hydrogen PeroxideTwo hydrogen (H) atoms can also bond with two oxygen (O) atoms, making the formula H2O2. That's just one more oxygen than water, but it is a totally different compound. You can see that each of the Oxygen atoms has eight electrons, and the two Hydrogens have two electrons each. See how the electrons are shared? |
|
|