
More About Equilibrium
Let's look at this equilibrium thing in a different way. Start with a table. There is a glass on the table. We'll pour a whole bunch of "X" into that glass. Eventually, some of that "X" breaks down into two pieces of "Y". That's one chemical reaction taking place.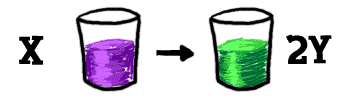
If you have another glass and you pour a bunch of "Y" into it, those "Ys" will eventually combine to make an "X". Using scientific terms, the "X" dissociates into two pieces of "Y", and the pieces of "Y" are going through a recombination to become "X".
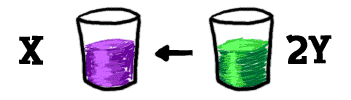
Now we have one glass with both reactions happening at the same time. If we look inside, the concentration of the molecules moves in one direction and then the other. Eventually, you won't see the concentrations change anymore. It's as if nothing is happening in the glass. That's equilibrium. The two reactions are still going on. They are just at a speed where they cancel each other out and you can see no change. The reactions are at a "happy" position.
The Position of Equilibrium
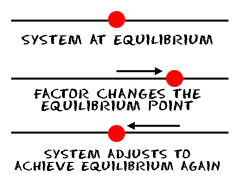 When a bunch of molecules are left alone, they reach a state of equilibrium. But that position of equilibrium can change if something happens to the molecules. Here's a list of things that can change the equilibrium point:
When a bunch of molecules are left alone, they reach a state of equilibrium. But that position of equilibrium can change if something happens to the molecules. Here's a list of things that can change the equilibrium point:
1. New molecules or substances are added that are not a part of the main reaction.
2. The temperature of the system is changed.
3. The pressure of the system is changed.
4. The concentrations are changed, like adding more water to a solution or adding more of one reactant or product.
5. There is a change in the total volume of the system.
Equilibrium doesn't always mean that there are equal numbers of reactant and product molecules. Our equilibrium point may look like it is in the middle of the two concentrations, but it can be anywhere. It's all about balance and finding a happy point. There are times when everything becomes a product, and other reactions where nothing happens. It all depends on the molecules and conditions of the system.
Le Chatelier, What Did He Say?
 There was a French guy named Henri Le Chatelier, and he came up with a principle for systems in equilibrium. The principle says that if you have a system in equilibrium and you do anything to it that messes up the equilibrium, the system will try to move back to the original state of equilibrium. Or, if you have a happy system and you make it unhappy, it will try to make itself happy again.
There was a French guy named Henri Le Chatelier, and he came up with a principle for systems in equilibrium. The principle says that if you have a system in equilibrium and you do anything to it that messes up the equilibrium, the system will try to move back to the original state of equilibrium. Or, if you have a happy system and you make it unhappy, it will try to make itself happy again.
His exact words were, "A system in equilibrium, when subjected to a stress resulting from a change in temperature, pressure, or concentration, and causing the equilibrium to be upset, will adjust its position of equilibrium to relieve the stress and reestablish equilibrium."
Learn more about equilibrium in part one.
► NEXT PAGE ON CHEMICAL REACTIONS
► EQUILIBRUM QUIZ
► RETURN TO TOP OF PAGE
► Or search the sites...
► EQUILIBRUM QUIZ
► RETURN TO TOP OF PAGE
► Or search the sites...
Related Video...
Biophysical Chemist: Profiles of Scientists (US-NSF Video)
Encyclopædia Britannica: Thermodynamic Equilibrium
Wikipedia: Chemical Equilibrium
Encyclopedia.com: Equilibrium



