
Measuring Reaction Rates
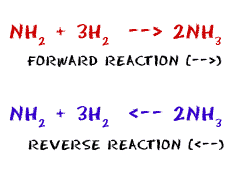 Scientists like to know the rates of reactions. They like to measure different kinds of rates too. Each rate that can be measured tells scientists something different about the reaction. We're going to take a little time to cover a few different measures of reaction rates.
Scientists like to know the rates of reactions. They like to measure different kinds of rates too. Each rate that can be measured tells scientists something different about the reaction. We're going to take a little time to cover a few different measures of reaction rates.
Forward Rate: The rate of the forward reaction when reactants combine to become products.
Reverse Rate: The rate of the reverse reaction when products break apart to become reactants.
Net Rate: The forward rate minus the reverse rate.
Average Rate: The speed of the entire reaction from start to finish.
Instantaneous Rate: The speed of the reaction at one moment in time. Some reactions can happen quickly at the start and then slow down. You have one average rate, but the instantaneous rates can tell you the whole story.
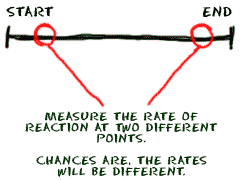 Scientists measure all of these rates by finding out the concentrations of the molecules in the mixture. If you find out the concentration of molecules at two different times, you can find out what direction the reaction is moving toward and how fast it is going. Even if the concentrations are equal at the two points of measurement, scientists still learn something. If the concentrations are stable during two measurements, the reaction is at an equilibrium point.
Scientists measure all of these rates by finding out the concentrations of the molecules in the mixture. If you find out the concentration of molecules at two different times, you can find out what direction the reaction is moving toward and how fast it is going. Even if the concentrations are equal at the two points of measurement, scientists still learn something. If the concentrations are stable during two measurements, the reaction is at an equilibrium point.
One Step at a Time
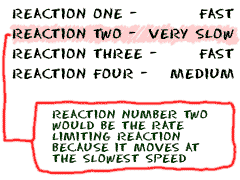 There is still more to know about measuring the rates of reactions. Since many reactions happen in several steps, the rate for each step needs to be measured. There will always be one step that happens at the slowest speed. That slowest step is called the rate-limiting step. That rate-limiting step is the one reaction that really determines how fast the overall reaction can happen. If you have six steps in your series of reactions and the third step goes incredibly slow, that is the rate-limiting step. As far as the overall reaction is concerned, none of the other rates really matter. If you want to speed up the overall reaction, you would focus on that slowest step. Don't forget that if you only speed up one step, another step may become the new rate-limiting step. You should always understand how all of the steps are involved in the overall reaction.
There is still more to know about measuring the rates of reactions. Since many reactions happen in several steps, the rate for each step needs to be measured. There will always be one step that happens at the slowest speed. That slowest step is called the rate-limiting step. That rate-limiting step is the one reaction that really determines how fast the overall reaction can happen. If you have six steps in your series of reactions and the third step goes incredibly slow, that is the rate-limiting step. As far as the overall reaction is concerned, none of the other rates really matter. If you want to speed up the overall reaction, you would focus on that slowest step. Don't forget that if you only speed up one step, another step may become the new rate-limiting step. You should always understand how all of the steps are involved in the overall reaction.
Related Video...
Strange Flames on the ISS (Science@NASA Video)
Encyclopædia Britannica: Chemical Kinetics
Wikipedia: Chemical Kinetics
Encyclopedia.com: Reaction Kinetics



