
Atoms Are Building Blocks
Atoms are the foundation of chemistry. They are the basis for everything in the Universe. As you know, matter is composed of atoms. Solids are made of densely packed atoms while gases have atoms that are spread out. We're going to cover basics like atomic structure and bonding between atoms. As you learn more, you can move to the reactions and biochemistry pages and see how atoms form compounds that help the biological world survive.
Are there pieces of matter that are smaller than atoms? Sure there are. Super-small particles can be found inside the pieces of atoms. These subatomic particles include nucleons and quarks. Nuclear chemists and physicists work together at particle accelerators to discover the presence of these tiny, tiny, tiny pieces of matter. However, science is based on the atom because it is the smallest distinct unit of matter.
Three Easy Pieces
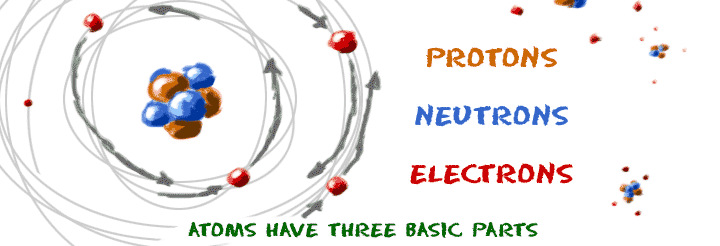
 Even though many super-tiny atomic particles exist, you only need to remember the three basic parts of an atom: electrons, protons, and neutrons. What are electrons, protons, and neutrons? Electrons are the smallest of the three particles that make up atoms. Electrons are found in shells or orbitals that surround the nucleus of an atom. Protons and neutrons are found in the nucleus. They group together in the center of the atom. That's all you have to remember. Three easy pieces!
Even though many super-tiny atomic particles exist, you only need to remember the three basic parts of an atom: electrons, protons, and neutrons. What are electrons, protons, and neutrons? Electrons are the smallest of the three particles that make up atoms. Electrons are found in shells or orbitals that surround the nucleus of an atom. Protons and neutrons are found in the nucleus. They group together in the center of the atom. That's all you have to remember. Three easy pieces!
There are almost 120 known elements in the periodic table. (117 as we write this) Chemists and physicists are trying to make new ones every day in their labs. The atoms of different elements have different numbers of electrons, protons, and neutrons. Every element is unique and has an atomic number. That number tells you the number of protons in every atom of the element. The atomic number is also called the proton number.
Charges of Atoms
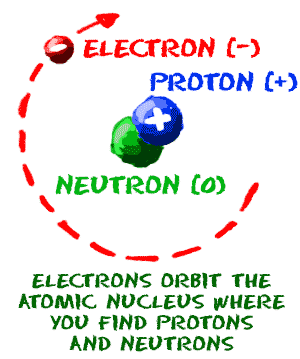
 You can see that each part of the atom is labeled with a "+", "-", or a "0." Those symbols refer to the charge of the particle. Have you ever heard about getting a shock from a socket, static electricity, or lightning? Those are all related to electric charges. Charges are also found in tiny particles of matter.
You can see that each part of the atom is labeled with a "+", "-", or a "0." Those symbols refer to the charge of the particle. Have you ever heard about getting a shock from a socket, static electricity, or lightning? Those are all related to electric charges. Charges are also found in tiny particles of matter.
The electron always has a "-", or negative, charge. The proton always has a "+", or positive, charge. If the charge of an entire atom is "0", or neutral, there are equal numbers of positive and negative charges. Neutral atoms have equal numbers of electrons and protons. The third particle is the neutron. It has a neutral charge, also known as a charge of zero.
Since the number of protons in an atom does not change, fewer or extra electrons can create a special atom called an ion. Cations have fewer electrons and have a positive charge. Anions have extra electrons that create a negative charge.
► NEXT PAGE ON ATOMS
► NEXT STOP ON SITE TOUR
► ATOMIC STRUCTURE QUIZ
► RETURN TO TOP OF PAGE
► Or search the sites...
► NEXT STOP ON SITE TOUR
► ATOMIC STRUCTURE QUIZ
► RETURN TO TOP OF PAGE
► Or search the sites...
Related Video...
The World’s Smallest Writing (Stanford Univ. Video)



