
Looking at Ions
 We've talked about ions before. Now it's time to get down to basics. The atomic number of an element, also called a proton number, tells you the number of protons or positive particles in an atom. A normal atom has a neutral charge with equal numbers of positive and negative particles. That means an atom with a neutral charge is one where the number of electrons is equal to the atomic number. Ions are atoms with extra electrons or missing electrons. When you are missing an electron or two, you have a positive charge. When you have an extra electron or two, you have a negative charge.
We've talked about ions before. Now it's time to get down to basics. The atomic number of an element, also called a proton number, tells you the number of protons or positive particles in an atom. A normal atom has a neutral charge with equal numbers of positive and negative particles. That means an atom with a neutral charge is one where the number of electrons is equal to the atomic number. Ions are atoms with extra electrons or missing electrons. When you are missing an electron or two, you have a positive charge. When you have an extra electron or two, you have a negative charge.
 What do you do if you are a sodium (Na) atom? You have eleven electrons — one too many to have an entire shell filled. You need to find another element that will take that electron away from you. When you lose that electron, you will you’ll have full shells. Whenever an atom has full shells, we say it is "happy." Let's look at chlorine (Cl). Chlorine has seventeen electrons and only needs one more to fill its third shell and be "happy." Chlorine will take your extra sodium electron and leave you with 10 electrons inside of two filled shells. You are now a happy atom too. You are also an ion and missing one electron. That missing electron gives you a positive charge. You are still the element sodium, but you are now a sodium ion (Na+). You have one less electron than your atomic number.
What do you do if you are a sodium (Na) atom? You have eleven electrons — one too many to have an entire shell filled. You need to find another element that will take that electron away from you. When you lose that electron, you will you’ll have full shells. Whenever an atom has full shells, we say it is "happy." Let's look at chlorine (Cl). Chlorine has seventeen electrons and only needs one more to fill its third shell and be "happy." Chlorine will take your extra sodium electron and leave you with 10 electrons inside of two filled shells. You are now a happy atom too. You are also an ion and missing one electron. That missing electron gives you a positive charge. You are still the element sodium, but you are now a sodium ion (Na+). You have one less electron than your atomic number.
Ion Characteristics
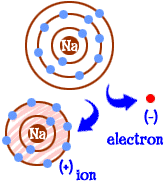 So now you've become a sodium ion. You have ten electrons. That's the same number of electrons as neon (Ne). But you aren't neon. Since you're missing an electron, you aren't really a complete sodium atom either. As an ion you are now something completely new. Your whole goal as an atom was to become a "happy atom" with completely filled electron shells. Now you have those filled shells. You have a lower energy. You lost an electron and you are "happy." So what makes you interesting to other atoms? Now that you have given up the electron, you are quite electrically attractive. Other electrically charged atoms (ions) of the opposite charge (negative) are now looking at you and seeing a good partner to bond with. That's where the chlorine comes in. It's not only chlorine. Almost any ion with a negative charge will be interested in bonding with you.
So now you've become a sodium ion. You have ten electrons. That's the same number of electrons as neon (Ne). But you aren't neon. Since you're missing an electron, you aren't really a complete sodium atom either. As an ion you are now something completely new. Your whole goal as an atom was to become a "happy atom" with completely filled electron shells. Now you have those filled shells. You have a lower energy. You lost an electron and you are "happy." So what makes you interesting to other atoms? Now that you have given up the electron, you are quite electrically attractive. Other electrically charged atoms (ions) of the opposite charge (negative) are now looking at you and seeing a good partner to bond with. That's where the chlorine comes in. It's not only chlorine. Almost any ion with a negative charge will be interested in bonding with you.
Electrovalence
Don't get worried about the big word. Electrovalence is just another word for something that has given up or taken electrons and become an ion. If you look at the periodic table, you might notice that elements on the left side usually become positively charged ions (cations) and elements on the right side get a negative charge (anions). That trend means that the left side has a positive valence and the right side has a negative valence. Valence is a measure of how much an atom wants to bond with other atoms. It is also a measure of how many electrons are excited about bonding with other atoms.
There are two main types of bonding, covalent and electrovalent. You may have heard of the term "ionic bonds." Ionic bonds are electrovalent bonds. They are just groups of charged ions held together by electric forces. Scientists call these groups "ionic agglomerates." When in the presence of other ions, the electrovalent bonds are weaker because of outside electrical forces and attractions. Sodium and chlorine ions alone have a very strong bond, but as soon as you put those ions in a solution with H+, OH-, F- or Mg++ ions, there are charged distractions that break the Na-Cl bond.

Look at sodium chloride (NaCl) one more time. Salt is a very strong bond when it is sitting on your table. It would be nearly impossible to break those ionic/electrovalent bonds. However, if you put that salt into some water (H2O), the bonds break very quickly. It happens easily because of the electrical attraction of the water. Now you have sodium (Na+) and chlorine (Cl-) ions floating around the solution. You should remember that ionic bonds are normally strong, but they are very weak in water.
► NEXT PAGE ON ATOMS
► NEXT STOP ON SITE TOUR
► ATOMIC STRUCTURE QUIZ
► RETURN TO TOP OF PAGE
► Or search the sites...
► NEXT STOP ON SITE TOUR
► ATOMIC STRUCTURE QUIZ
► RETURN TO TOP OF PAGE
► Or search the sites...
Related Video...
NEXT Ion Propulsion System (NASA Video)



