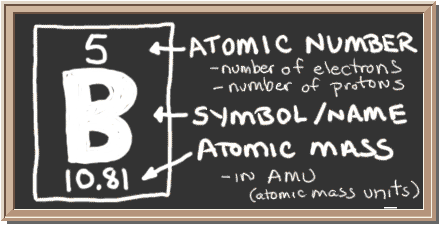
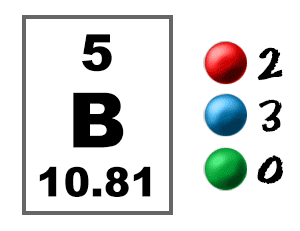
Check out the blackboard. That box on the left has all of the information you need to know about one element. It tells you the mass of one atom, how many pieces are inside, and where it should be placed on the periodic table.
In the next section we're going to cover electron orbitals or electron shells. This may be a new topic to some of you.
Electrons In The Shells
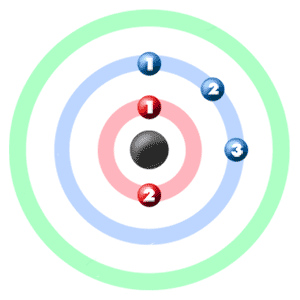
Take a look at the picture below. Each of those colored balls is an electron. In an atom, the electrons spin around the center, also called the nucleus. The electrons like to be in separate shells/orbitals. Shell number one can only hold 2 electrons, shell two can hold 8, and for the first eighteen elements shell three can hold a maximum of eight electrons. As you learn about elements with more than eighteen electrons you will find that shell three can hold more than eight. Once one shell is full, the next electron that is added has to move to the next shell.So... for the element of BORON, you already know that the atomic number tells you the number of electrons. That means there are 5 electrons in an boron atom. Looking at the picture, you can see there are two electrons in shell one and three more in shell two.
Examples of Compounds with Boron
Boron TrichlorideBoron (B) is able to form compounds with chlorine (Cl) to create BCl3. Because of it's structure, boron is able to share it's three extra electrons with three separate chlorine atoms. If you look at the dot structure, you'll see that each of the chlorine atoms has eight electrons, making their shells full! |
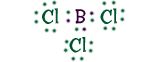 
|
|
|
|
||
BoraneBorane is the name scientists have when one boron (B) atom bonds to three hydrogen (H) atoms. You can see that each of the hydrogen atoms now has two electrons, filling their outer shell. The boron atom has lost its three extra electrons, giving it a full shell as well. |
|
|