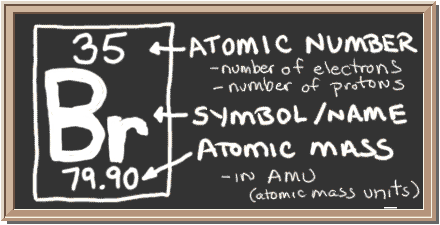
Check out the blackboard. That box on the left has all of the information you need to know about one element. It tells you the mass of one atom, how many pieces are inside, and where it should be placed on the periodic table.
Now we're working with the fourth period/row in the table of elements. You may have an easy way to know the number of electrons in a neutral atom, but the placement of those electrons gets a little more complex. Let's take a look at the arrangements of electrons in the basic elements (left and right sides of the table) of period four and the more complex arrangements of the transition elements (in the middle of the row). If you think this is a little over your head, go back and look at the elements 1-18 that have organizations that are a little more simple.
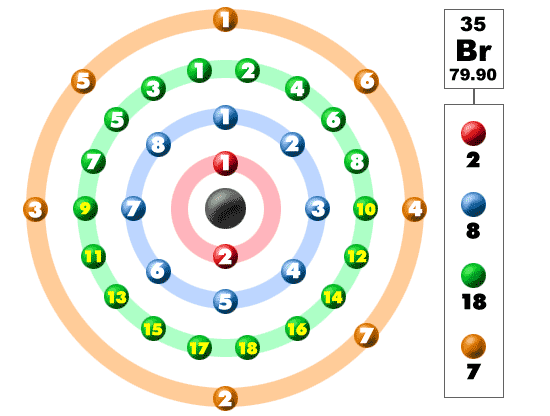
Electrons In The Shells
Take a look at the picture below. Each of those colored balls is an electron. In an atom, the electrons spin around the center, also called the nucleus. The electrons like to be in separate shells/orbitals. As you learn more about atomic structure, you will learn that the electrons don't stay in defined areas around the nucleus. They are found in clouds that can have different shapes that include spheres and dumbbell-like shapes. So remember when you look at our breakdown that the electrons aren't always in a nice neat order as shown here.Bromine is a member of the halogen family of elements. Its companions include fluorine, chlorine, and iodine. Like the other halogens, bromine has seven electrons in its outer shell and is very reactive. You will find bromine in many salt compounds with alkali metals. Sodium bromide is a compound found in seawater. As with all reactive elements, bromine is never found alone in nature. It is always a part of a compound with other elements.