
Changing States of Matter
All matter can move from one state to another. It may require extreme temperatures or extreme pressures, but it can be done. Sometimes a substance doesn't want to change states. You have to use all of your tricks when that happens. To create a solid, you might have to decrease the temperature by a huge amount and then add pressure. For example, oxygen (O2) will solidify at -361.8 degrees Fahrenheit (-218.8 degrees Celsius) at standard pressure. However, it will freeze at warmer temperatures when the pressure is increased.
Some of you know about liquid nitrogen (N2). It is nitrogen from the atmosphere in a liquid form and it has to be super cold to stay a liquid. What if you wanted to turn it into a solid but couldn't make it cold enough to solidify? You could increase the pressure in a sealed chamber. Eventually you would reach a point where the liquid became a solid. If you have liquid water (H2O) at room temperature and you wanted water vapor (gas), you could use a combination of high temperatures or low pressures to solve your problem.
Points of Change
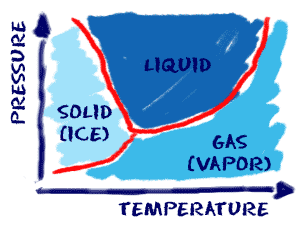 Phase changes happen when you reach certain special points. Sometimes a liquid wants to become a solid. Scientists use something called a freezing point or melting point to measure the temperature at which a liquid turns into a solid. There are physical effects that can change the melting point. Pressure is one of those effects. When the pressure surrounding a substance increases, the freezing point and other special points also go up. It is easier to keep things solid when they are under greater pressure.
Phase changes happen when you reach certain special points. Sometimes a liquid wants to become a solid. Scientists use something called a freezing point or melting point to measure the temperature at which a liquid turns into a solid. There are physical effects that can change the melting point. Pressure is one of those effects. When the pressure surrounding a substance increases, the freezing point and other special points also go up. It is easier to keep things solid when they are under greater pressure.
Generally, solids are more dense than liquids because their molecules are closer together. The freezing process compacts the molecules into a smaller space.
There are always exceptions in science. Water is special on many levels. It has more space between its molecules when it is frozen. The molecules organize in a specific arrangement that takes up more space than when they are all loosey-goosey in the liquid state. Because the same number of molecules take up more space, solid water is less dense than liquid water. There are many other types of molecular organizations in solid water than we can talk about here.
| CHEMISTRY TERM | PHASE CHANGE |
|
Fusion/Melting Freezing Vaporization/Boiling Condensation Sublimation Deposition |
Solid to a Liquid Liquid to a Solid Liquid to a Gas Gas to a Liquid Solid to a Gas Gas to a Solid |
Solid to a Liquid and Back to a Solid
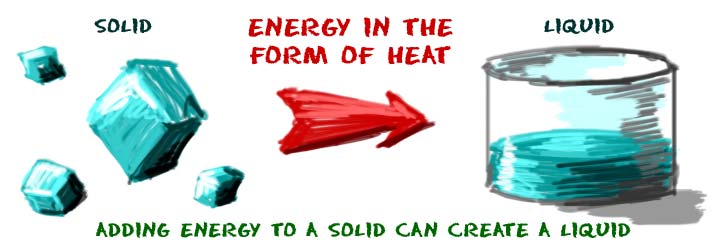
Imagine that you are a solid. You're a cube of ice sitting on a counter. You dream of becoming liquid water. You need some energy. Heat is probably the easiest energy you can use to change your physical state. The atoms in a liquid have more energy than the atoms in a solid.
There is a special temperature for every substance called the melting point. When a solid reaches the temperature of its melting point, it can become a liquid. For water, the temperature needs to be a little over zero degrees Celsius (0oC) for you to melt.
If you were salt, sugar, or rock, your melting point is higher than that of water. How do you know that fact? If their melting points were lower, they would also be liquids when the temperature is above zero degrees Celsius. The reverse of the melting process is called freezing. Liquid water freezes and becomes solid ice when the molecules lose energy.
Solid to a Gas and Back to a Solid
You know about solids melting and becoming liquids. Some of you may have also seen a solid become a gas. It's a process called sublimation. The easiest example of sublimation might be dry ice. Dry ice is solid carbon dioxide (CO2). Amazingly, when you leave dry ice out in a room, it just turns into a gas. Have you ever heard of liquid carbon dioxide? It can be made, but not in normal situations. Coal is another example of a compound that will not melt at normal atmospheric pressures. It will sublimate at very high temperatures.
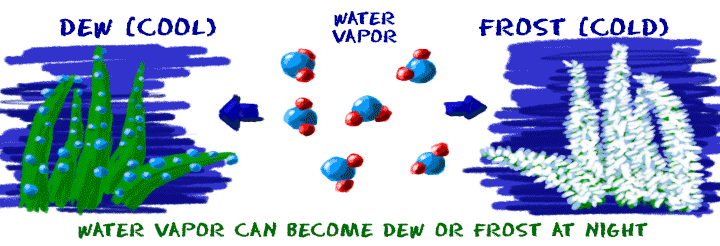
Can you go from a gas to a solid? Sure. Deposition occurs when a gas becomes a solid without going through the liquid state of matter. Those of you who live near the equator may not have seen it, but closer to the poles we see frost on winter mornings. Those little frost crystals on plants build up when water vapor from the air becomes a solid on the leaves of plants.
► More on Phase Changes in Part II...
Related Video...
Liquid Onboard the ISS (NASA/MSFC Video)
Encyclopædia Britannica: Phase Transitions
Wikipedia: Phase Changes
Wikipedia: Phase Transitions
Encyclopedia.com: States of Matter
University of Colorado: States of Matter
UC Davis: Phase Trasitions



