
Chemical Changes Versus Physical Changes
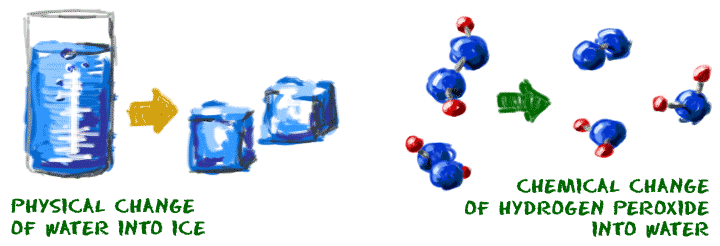
It is important to understand the difference between chemical and physical changes. Some changes are obvious, but there are some basic ideas you should know. Physical changes are usually about physical states of matter. Chemical changes happen on a molecular level when you have two or more molecules that interact. Chemical changes happen when atomic bonds are broken or created during chemical reactions.
No Change to Molecules
When you step on a can and crush it, you have forced a physical change. However, you only changed the shape of the can. It wasn't a change in the state of matter because the energy in the can did not change. Also, since this was a physical change, the molecules in the can are still the same molecules. No chemical bonds were created or broken.
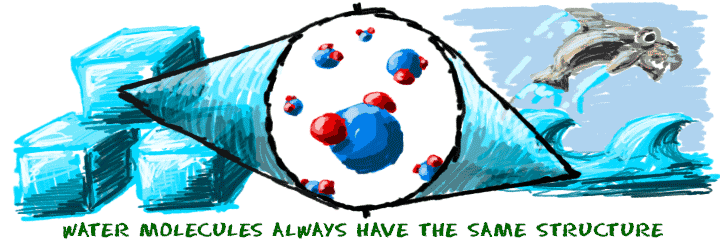
When you melt an ice cube (H2O), you have a physical change because you add energy. You added enough energy to create a phase change from solid to liquid. Physical actions, such as changing temperature or pressure, can cause physical changes. No chemical changes took place when you melted the ice. The water molecules are still water molecules.
Changing the Molecules
Chemical changes happen on a much smaller scale. While some experiments show obvious chemical changes, such as a color change, most chemical changes are not visible. The chemical change as hydrogen peroxide (H2O2) becomes water cannot be seen since both liquids are clear. However, behind the scenes, billions of chemical bonds are being created and destroyed. In this example, you may see bubbles of oxygen (O2) gas. Those bubbles are evidence of the chemical changes.
Melting a sugar cube is a physical change because the substance is still sugar. Burning a sugar cube is a chemical change. Fire activates a chemical reaction between sugar and oxygen. The oxygen in the air reacts with the sugar and the chemical bonds are broken.
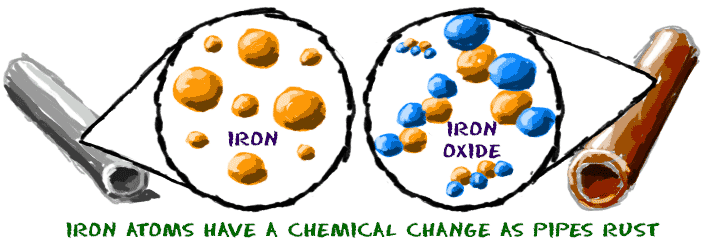
Iron (Fe) rusts when it is exposed to oxygen gas in the air. You can watch the process happen over a long period of time. The molecules change their structure as the iron is oxidized, eventually becoming iron oxide (Fe2O3). Rusty pipes in abandoned buildings are real world examples of the oxidation process.
Isomers
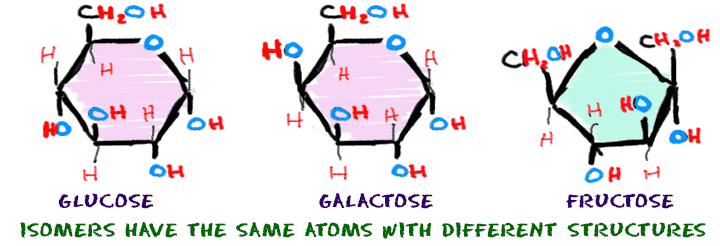 Some chemical changes are extremely small and happen over a series of steps. The resulting compounds might have the same number of atoms, but they will have a different structure or combination of atoms.
Some chemical changes are extremely small and happen over a series of steps. The resulting compounds might have the same number of atoms, but they will have a different structure or combination of atoms.
The sugars glucose, galactose, and fructose all have six carbon atoms, twelve hydrogen atoms, and six oxygen atoms (C6H12O6). Even though they are made of the same atoms, they have very different shapes and are called isomers. Isomers have atoms bonded in different orders.
Each of the sugars goes through different chemical reactions because of the differences in their molecular structure. Scientists say that the arrangement of atoms allows for a high degree of specificity, especially in the molecules of living things. Specificity means the molecules will only work in specific reactions, not all of them. For example, your body uses glucose as an energy source. If you eat galactose molecules, they need to be converted into glucose before your body can use them.
Related Video...
Recipes for Chemists (NASA/NASA Connect Video)
Encyclopædia Britannica: Phases of Matter
Wikipedia: Physical Changes
Wikipedia: Chemical Changes
Encyclopedia.com: Chemical Bonding
UC Davis: Chemical vs Physical
Rhode Island College: Chemical Changes



