
Evaporation of Liquids
Sometimes a liquid can be sitting in one place (maybe a puddle) and its molecules will become a gas. That's the process called evaporation. It can happen when liquids are cold or when they are warm. It happens more often with warmer liquids. You probably remember that when matter has a higher temperature, the molecules have a higher energy. When the energy in specific molecules reaches a certain level, they can have a phase change. Evaporation is all about the energy in individual molecules, not about the average energy of a system. The average energy can be low and the evaporation still continues.
You might be wondering how that can happen when the temperature is low. It turns out that all liquids can evaporate at room temperature and normal air pressure. Evaporation happens when atoms or molecules escape from the liquid and turn into a vapor. Not all of the molecules in a liquid have the same energy. When you have a puddle of water (H2O) on a windy day, the wind can cause an increased rate of evaporation even when it is cold out.
Energy Transfer
The energy you can measure with a thermometer is really the average energy of all the molecules in the system. There are always a few molecules with a lot of energy and some with barely any energy at all. There is a variety, because the molecules in a liquid can move around. The molecules can bump into each other, and when they hit... Blam! A little bit of energy moves from one molecule to another. Since that energy is transferred, one molecule will have a little bit more and the other will have a little bit less. With trillions of molecules bouncing around, sometimes individual molecules gain enough energy to break free. They build up enough power to become a gas once they reach a specific energy level. In a word, when the molecule leaves, it has evaporated.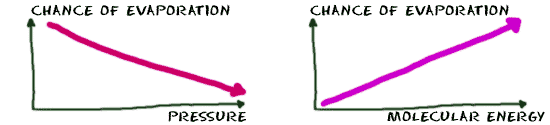 The rate of evaporation can also increase with a decrease in the gas pressure around a liquid. Molecules like to move from areas of higher pressure to lower pressure. The molecules are basically sucked into the surrounding area to even out the pressure. Once the vapor pressure of the system reaches a specific level, the rate of evaporation will slow down.
The rate of evaporation can also increase with a decrease in the gas pressure around a liquid. Molecules like to move from areas of higher pressure to lower pressure. The molecules are basically sucked into the surrounding area to even out the pressure. Once the vapor pressure of the system reaches a specific level, the rate of evaporation will slow down.
Related Video...
The Evaporative Stress Index (NASA/GSFC Video)



