
Stoichiometry
 Let's start with how to say this word. Five syllables: STOY-KEE-AHM-EH-TREE. It's a big word that describes a simple idea. Stoichiometry is the part of chemistry that studies amounts of substances that are involved in reactions. You might be looking at the amounts of substances before the reaction. You might be looking at the amount of material that is produced by the reaction. Stoichiometry is all about the numbers.
Let's start with how to say this word. Five syllables: STOY-KEE-AHM-EH-TREE. It's a big word that describes a simple idea. Stoichiometry is the part of chemistry that studies amounts of substances that are involved in reactions. You might be looking at the amounts of substances before the reaction. You might be looking at the amount of material that is produced by the reaction. Stoichiometry is all about the numbers.
All reactions are dependent on how much stuff you have. Stoichiometry helps you figure out how much of a compound you will need, or maybe how much you started with. We want to take the time to explain that reactions depend on the compounds involved and how much of each compound is needed.
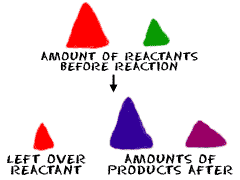
 What do you measure? It could be anything. When you're doing problems in stoichiometry, you might look at...
What do you measure? It could be anything. When you're doing problems in stoichiometry, you might look at...- Mass of Reactants (chemicals before the reaction)
- Mass of Products (chemicals after the reaction)
- Chemical Equations
- Molecular Weights of Reactants and Products
- Formulas of Various Compounds
Now, an example. Let's start with something simple like sodium chloride (NaCl). You start with two ions and wind up with an ionic/electrovalent compound. When you look at the equation, you see that it takes one sodium ion (Na+) to combine with one chlorine ion (Cl-) to make the salt. When you use stoichiometry, you can determine amounts of substances needed to fulfill the requirements of the reaction. Stoichiometry will tell you that, if you have ten million atoms of sodium and only one atom of chlorine, you can only make one molecule of sodium chloride. Nothing you can do will change that. It's like this:
10,000,000 Na + 1 Cl --> NaCl + 9,999,999 Na
 Let's bump it up a level. When you mix hydrogen gas (H2) and oxygen gas (O2), nothing much happens. When you add a spark to the mixture, all of the molecules combine and eventually form water (H2O). You would write it like this:
Let's bump it up a level. When you mix hydrogen gas (H2) and oxygen gas (O2), nothing much happens. When you add a spark to the mixture, all of the molecules combine and eventually form water (H2O). You would write it like this:
2H2 + O2 --> 2H2O
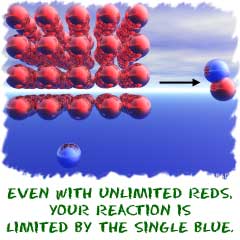
What does stoichiometry look at here? First, look at the equation. Four hydrogen atoms and two oxygen atoms are on each side of the equation. It's an important idea to see that you need twice as many hydrogen atoms as you do oxygen atoms. The number of atoms in the equation will help you figure out how much of each substance you will need to make the reaction happen. If you make this an extreme example and fill a sealed container with one million hydrogen molecules and only one oxygen molecule, the spark won't make an explosion. There is no monster reaction to be created when there is only one oxygen molecule around. You will make two water molecules and be done.
Related Video...
Building a Career at NASA (NASA/Glenn Video)
Encyclopædia Britannica: Stoichiometry
Wikipedia: Stoichiometry
Encyclopedia.com: Chemical Equations



